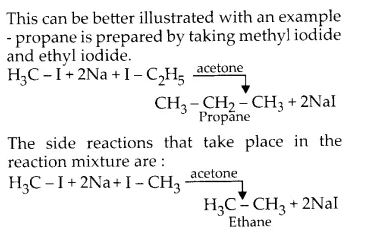Chapter 13 : Hydrocarbons
chemistry
NCERT Exercises

Question 1.
How do you account for the formation of ethane during chlorination of methane?
Solution.
This is how ethane is generated during the chlorination of methane.



Question 2.
Write IUPAC names of the following compounds:
Solution.


Question 3.
For the following compounds, write structural formulae and IUPAC names of all possible isomers having the number of double or triple bond as indicated:
(a) C4H8 (one double bond)
(b) C5H8 (one triple bond)
Solution.


Question 4.
Write IUPAC names of the products obtained by the ozonolysis of the following compounds:
(i) pent-2-ene
(ii) 3,4-dimethylhept-3-ene
(iii) 2-ethylbut-1-ene
(iv) 1-phenylbut-1-ene
Solution.

Question 5.
An alkene A on ozonolysis gives a mixture of ethanal and pentan-3-one. Write the structure and IUPAC name of A.
Solution.


Question 6.
An alkene A contains three C – C, eight C – H σ bonds and one C – C π bond. A on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write the IUPAC name of A.
Solution.

Question 7.
Propanal and pentan-3-one are the ozonolysis products of an alkene. What is the structural formula of the alkene?
Solution.

Question 8.
Write chemical equations for combustion reaction of the following hydrocarbons:
(i) Butane
(ii) Pentene
(iii) Hexyne
(iv) Toluene
Solution.

Question 9.
Draw the cis and trans structures of hex-2-ene. Which isomer will have higher boiling point and why?
Solution.
Of the given isomers, the cis isomer has a higher boiling point. This difference arises due to higher dipole moment of the cis isomer which introduces a somewhat ionic character in the compound. In the trans isomer, the dipoles cancel each other resulting in a small dipole moment as the case may be.
Question 10.
Why is benzene extraordinary stable though it contains three double bonds?
Solution.
- The extraordinary stability of benzene molecule may be attributed to resonance in the molecule. In benzene, each of the 6 C atoms is sp2 hybridised with one p-orbital on each carbon atom left unhybridised. While 2 of the sp2 orbitals form bonds with 2 C-atoms, the third one is involved in bonding with hvdrogen atom. Thus, 3 of the valencies of C are satisfied.
- This leaves the unhybridised p-orbital containing 1 electron each for bonding. Each of these p-orbitals can overlap with the adjacent C atom and thus, results in bonding.
- Since the probability for each p-orbital to overlap with either of the two immediate neighbours is equal. Therefore, it alternately does so.
- Thus, the π electrons (in unhybridised p-orbitals) are no more localised between just 2 carbon atoms but these 671 electrons are shared or attracted by all the 6 carbon atoms.
- This increased attraction is the reason for the ‘extraordinary’ stability of the benzene molecule with 3 double bonds.
- These 3 π-bonds are not localised but are spread over the entire molecule.
Question 11.
What are the necessary conditions for any system to be aromatic?
Solution.
The necessary and sufficient condition for any system to be aromatic is given by Huckel’s rule. As per Huckel’s rule, any system is said to be aromatic if it satisfies the following 3 conditions:
- Contains (4n + 2) 71 electrons, where n is any positive integer or 0,
- Shows complete delocalisation of π electrons and
- The molecule must be planar.


Question 12.
Explain why the following systems are not aromatic?
Solution.
One of the conditions stated by the Huckel’s rule for any system to be aromatic is that of planarity i.e., all atoms of the molecule must be present on the same plane. This rule is violated in structure (i) and (ii). The carbon atom indicated below are sp3 hybridised which disallow planarity (sp3 hybridised carbon is tetrahedral in geometry).
In (iii) the number of n electrons is 8. (2 per double bond). The Huckel’s rule allows 2, 6,10,14, … etc. u electrons for any aromatic system. Since (iii) does not have (4n + 2) 7i electrons, therefore, it is not aromatic.


Question 13.
How will you convert benzene into
(i) p-nitrobromobenzene
(ii) m-nitrochlorobenzene
(iii) p-nitrotoluene
(iv) acetophenone
Solution.


Question 14.
Solution.
Question 15.
What effect does branching of an alkane chain has on its boiling point?
Solution.
As the branching in an alkane increases, the shape of the molecule approaches a sphere and size of the branched chain alkane becomes less than that of its straight chain counterpart. The reduced surface area results in decreased van der Waals’ interaction and finally leads to lower boiling point as compared to straight chain alkanes of comparable molar mass.




Question 16.
Addition of HBr to propene yields 2-bromopropane, while in the presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give mechanism.
Solution.

Question 17.
Write down the products of ozonolysis of 1,2-dimethylbenzene (o-xylene). How does the result support Kekule structure for benzene?
Solution.



Question 18.
Arrange benzene, n-hexane and ethyne in decreasing order of acidic behaviour. Also give reason for this behaviour.
Solution.

Question 19.
Why does benzene undergo electrophilic substitution reactions easily and nucleophilic substitutions with difficulty?
Solution.
Electrophiles are species that are electron deficient and hence, seek electron rich molecules. Benzene is one such molecule which is rich in electrons. It is so because benzene has 6n electrons delocalised over the entire molecule which acts as a good host for electrophiles.
Another point working in favour of electrophilic substitution reactions is the retention of aromaticity. A benzene molecule is highly stable owing to its aromatic character.
Therefore, it would not want to lose its aromaticity. Upon undergoing electrophilic substitution reaction, this aromaticity is not lost, it is retained and hence, benzene undergoes electrophilic substitution.
Contrast this with a nucleophilic substitution reaction where the nucleophile attacks. A nucleophile (Nu–) is a species that seeks a positive centre or an electron deficient species. Obviously, benzene is not electron deficient and therefore, will not be a welcome site for a Nu–. This is the major reason why benzene does not undergo a nucleophilic substitution reaction.
Another reason working against these reactions is the difficulty with which the transition state is formed. The transition state benzyne involved here is formed with great difficulty and hence, these reactions are difficult to bring about.

Question 20.
How would you convert the following compounds into benzene?
(i) Ethyne
(ii) Ethene
(iii) Hexane
Solution.

Question 21.
Write structures of all the alkenes which on hydrogenation give 2-methylbutane.
Solution.

Question 22.
Arrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, E+.
(a) Chlorobenzene, 2,4-dinitrochlorobenzene, p-nitrochlorobenzene
(b) Toluene, p-H3C – C6H4 – NO2, p-O2N – C6H4 – NO2
Solution.

Question 23.
Out of benzene, m-dinitrobenzene and toluene which will undergo nitration most easily and why?
Solution.
Question 24.
Suggest the name of a Lewis acid other than anhydrous aluminium chloride which can be used during ethylation of benzene.
Solution.
Other Lewis acids besides anhyd. AlCl3 that may be used during ethylation of benzene is anhy. FeCl3.
Question 25.
Why is Wurtz reaction not preferred for the preparation of alkanes containing odd number of carbon atoms? Illustrate your answer by taking one example.
Solution.
Wurtz reaction is used for preparation of alkanes. During this reaction, an alkyl halide with half the number of carbon atoms than the desired alkane is made to react with sodium metal in acetone. This leads to the formation of the desired alkane. e.g., If the desired alkane is ethane, methyl iodide is taken.
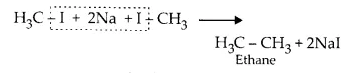
While this method is highly successful for producing alkanes with even number of carbon atoms but it gives a mixture of alkanes when odd numbered alkanes are to be formed. This happens because two different alkyl halides not only react with each other but also with themselves.